| Current Members |
| - Dr. Mark Thompson |
|
| - Judy Fong |
|
| - Dr. Peter Djurovich |
|
|
| - Dr. Sunil Kumar Kandappa |
| - Dr. Eric T. McClure |
| - Dr. Marsel Shafikov |
|
| - Megan Cassingham |
| - Gemma Goh |
| - Jie Ma |
| - Konstantin Mallon |
| - Junru Su |
| - Austin Mencke |
| - Collin Muniz |
| - James Fortwengler |
| - Chandler Dobson |
| - Mattia Di Niro |
| - Frances Yau |
| - Mahsa Rezaiyan |
| - Jonas Schaab |
| - Darius Allen Shariaty |
tr>
- Nina Baluyot-Reyes |
- Kelly Biv |
- Ao Jiao |
|
| - Claire Archer |
Biotic/Abiotic Interfaces
Background -- Biotic/Abiotic Interface:
Across all engineering disciplines, the need for materials capable of responding to external stimuli opens doors to more advanced and often more elegant solutions. These smart materials respond to one or multiple factors in their environment and thereby allow technologies that are more capable of producing desired results. Common among such materials are responsiveness to magnetic fields, pH, light, stress, voltage, and temperature, each allowing for different information to be read from a system and incorporated into device response. These properties have led to high accuracy sensors, materials that are malleable without contact, more advanced actuators, and other revolutionary technologies. The recent focus of this group has been the development of customized thermos-responsive materials to meet the technological shortcomings of current solutions to modern challenges.
Thermo-responsive materials offer a new trajectory for many fields, but particularly in the realm of biomedical applications. Invasive surgical methods increase the chance of complications and patient harm. Minimally invasive alternatives to standard surgical procedures receive increasing attention as they promise next generation of medical care standards. Wound closure or repair by glues with minimal physiological foreign body response—bioadhesives—can lower patient discomfort, and provide a more easily applied alternative to sutures or other conventional stitching methods. Furthermore, through examination of particle aggregation, elasticity, and viscosity, novel materials can be tuned to mimic the biomechanical properties of physiological tissue. As such, potential applications range from tissue repair to biomimicry. These therapies can be made even more adaptable by engineering the reversal of the gel transition and customizable critical temperatures. Beyond wound closures, advances can also be made when these materials are applied to drug delivery, tissue repair, and other more advanced designations.
Previous work has explored the use of custom copolymers of the well-known thermo-responsive Poly(N-isopropylacrylamide) (PNIPAM). PINAPAM is a smart polymer system investigated for a range of biomedical, drug screening, biotechnology and medical diagnostics applications. Below approximately 32-33°C (lower critical solution temperature, LCST), PNIPAM’s hydrophilic interactions with water enable a translucent liquid state. Above its LCST, it forms solid aggregate that readily adheres to tissues. 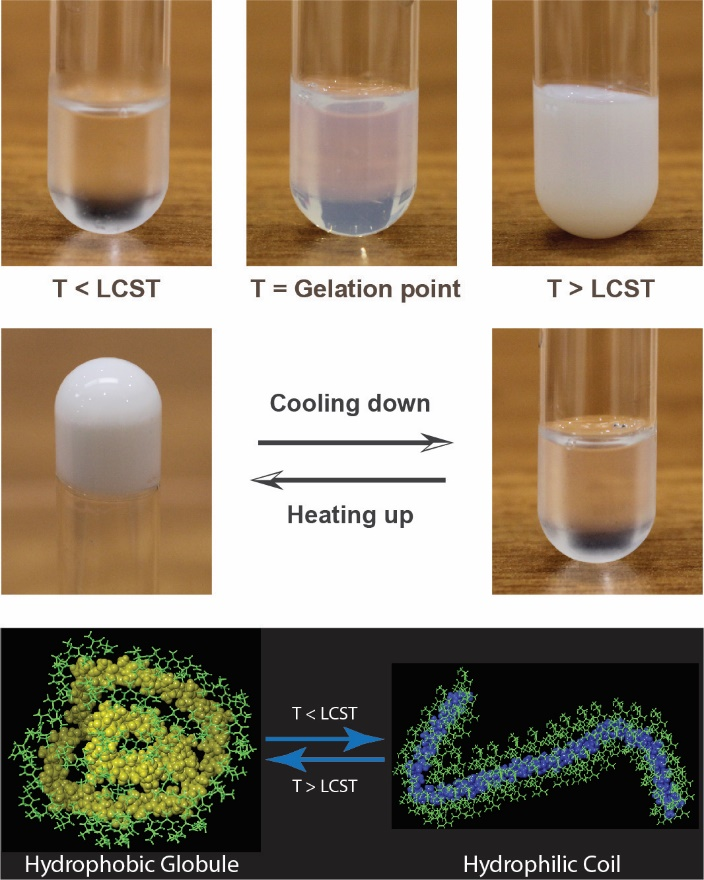 Through co-polymerization with other monomers (e.g. N-tert-butylacrylamide, butylacrylate) that behavior can be shifted such that the material forms a solid adhesive at physiologically relevant temperatures (Fig. 1). Through co-polymerization with other monomers (e.g. N-tert-butylacrylamide, butylacrylate) that behavior can be shifted such that the material forms a solid adhesive at physiologically relevant temperatures (Fig. 1).
Recent work focused on the development a biocompatible, tissue repair technology for temporary intervention at sites of scleral tissue damage or loss. Exploiting the co-polymerization properties mentioned above, we tailored this behavior to create a hydrogel to seal irregular margins of traumatic injuries. In particular, injection of a liquid sealant with body-heat induced gelation created a size-adaptable solidified occlusion. This technology was well suited for to the treatment of open globe eye injuries, for which current treatment options require use of microsurgical instrumentation accompanied by surgical microscopes to visualize tissue repair. In contrast, the developed material both restored intraocular pressure (IOP) and was easily removed without tissue damage. Implementation of this technology and others like it afford the patient a larger window of time to complete surgical intervention without requiring specialty equipment such as surgical microscopes for implementation. A simple design and easily applied form also cuts operation complexity and thereby time (Fig. 2).
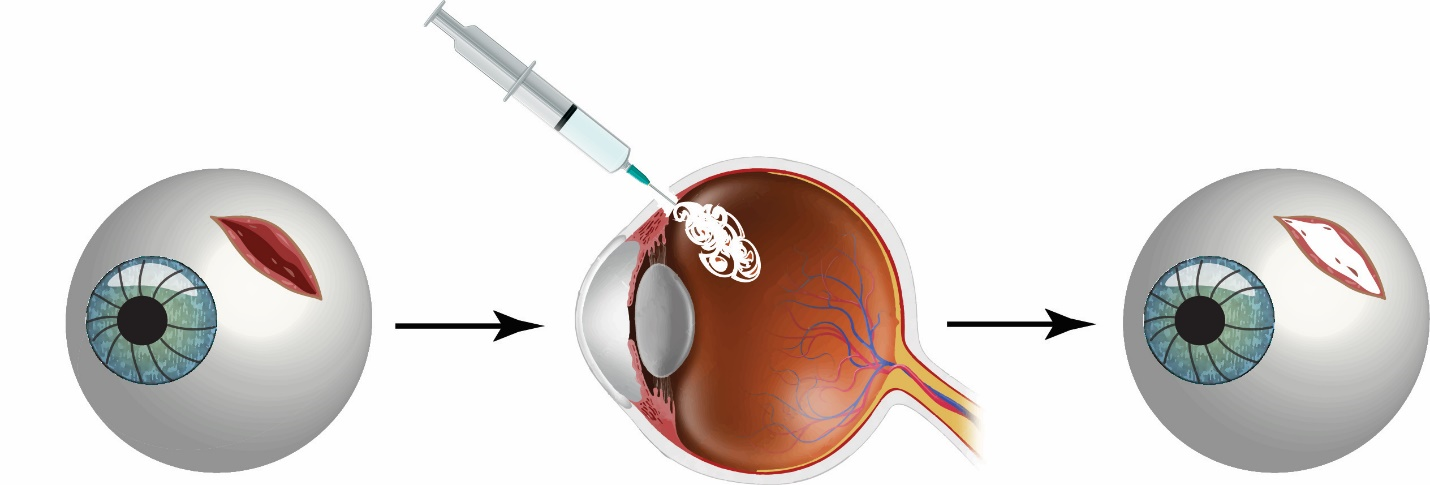
This project seeks to design innovative biomaterials and systems of delivery to improve medical standards and revolutionize the way we heal.
|

